Subscribe to our newsletter now!
Newsletter
News
8 December 2025
Merry Christmas and a Happy New Year from the SCTO
As 2025 comes to an end, we look back on a vibrant year for the SCTO. We entered a new funding period, set new strategic goals, and welcomed our new President. Our first Research-on-Research projects are underway, the SCTO Platforms released new templates, resources, and courses for clinical researchers, and we successfully held both the SCTO Forum and the SCTO Symposium. Simultaneously, our fully redesigned website is shaping up well and moving steadily toward launch.
In January 2026, we will proudly unveil our refreshed visual identity, featuring a redesigned logo, updated corporate design, and a modern website that brings together the content of scto.ch, sctoplatforms.ch, and cr-careers.ch. This new website will make it even easier to access tools, resources, and training opportunities across our SCTO Network, SCTO Platforms, and stakeholder community.
We wish you a wonderful Christmas season and a joyful start to 2026. May the new year bring you health, happiness, and success.
News
4 November 2025
Coming soon: The SCTO’s new website
We are excited to announce that the SCTO will soon be launching its new website, featuring a fresh logo and an updated look that reflect our evolving identity.
The websites www.sctoplatforms.ch and www.cr-careers.ch will be merged with our main site www.scto.ch, which will have a new design, improved navigation, and easier access to key information for a more integrated user experience.
The SCTO’s new website will also include two helpful new features: a Tool Finder for quickly locating relevant clinical research tools and resources and a Course Finder for identifying suitable training opportunities for clinical researchers.
Stay tuned for the launch of the SCTO’s new website this December!
News
2 July 2025
Latest publication from DCR Bern: An approach to implementing patient and public involvement in investigator-initiated clinical trials
As interest in patient and public involvement (PPI) grows in academic clinical research, this Viewpoint publication by the Department of Clinical Research (DCR) Bern explores how to build a basic framework for effective collaboration in Switzerland. The authors highlight key insights for preparing researchers and PPI contributors to work together.
News
12 June 2025
SCTO Symposium 2025
Bridging the divide: Integrating clinical trials with medical care
On 3 June 2025, more than 160 participants gathered in Basel for this year’s SCTO Symposium co-organised by the Department of Clinical Research, University of Basel and the Swiss Clinical Trial Organisation (SCTO).
Held under the theme Bridging the divide: Integrating clinical trials with medical care, the event brought together experts and stakeholders from across Switzerland to explore one of the most pressing challenges in clinical research today.
Discover the photo gallery and download the presentation slides here.
News
20 May 2025
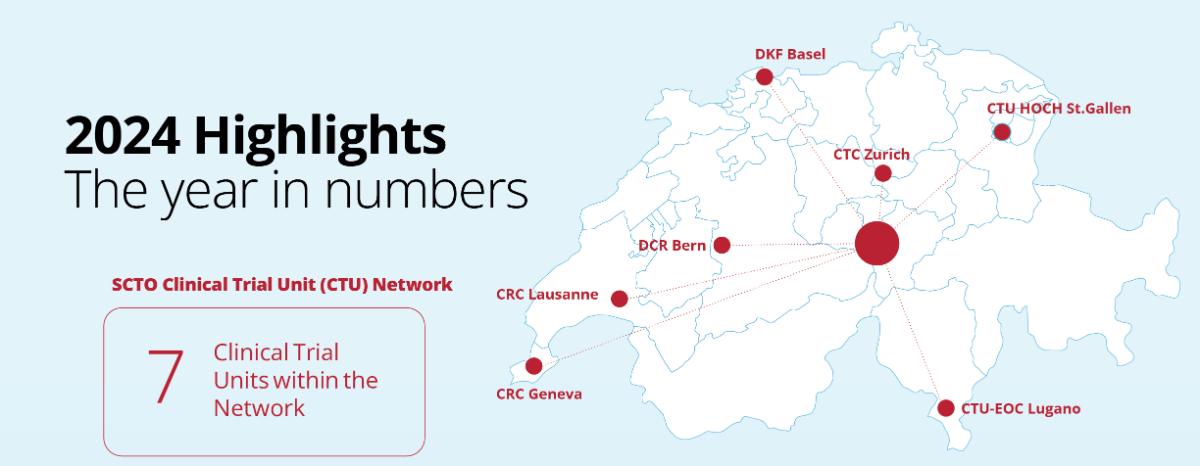
SCTO Annual Report 2024: A year of milestones and new beginnings
We invite you to explore the SCTO Annual Report 2024 and dive into the key achievements of our SCTO Clinical Trial Unit (CTU) Network and SCTO Platforms. The report highlights our efforts to strengthen harmonised clinical research through tools and resources for the clinical research community, our focus on patient and public involvement (PPI), and how we’ve engaged stakeholders through strategic communication and dialogue.
The year 2024 marks a major milestone: The successful conclusion of the 2021–2024 funding period. Over these years, the SCTO has made significant strides in advancing and harmonising high-quality clinical research in Switzerland—driven by collaboration, innovation, and education.
Looking ahead, we’re proud to share that the SCTO has secured renewed funding from the Swiss National Science Foundation (SNSF) and the State Secretariat for Education, Research and Innovation (SERI) for the 2025–2028 period. This continued support paves the way for new strategic directions in a dynamic clinical research environment.

News
20 May 2025
A new era for the SCTO: Welcoming our new President, Prof. Dr. med. Alessandro Ceschi, M.Sc.
On Tuesday, 13 May 2025, the SCTO General Meeting of Members elected the SCTO organs for the 2025–2028 period, including a new President and a Steering Board member. During the General Assembly, a review of the SCTO’s 2024 activities, a look ahead to the new funding period, and updates from key stakeholders have been presented.
After seven years of dedicated leadership by Prof. Dr. med. Christiane Pauli-Magnus, Co-Director of the Department of Clinical Research at University Hospital Basel, the General Meeting of Members has elected Prof. Dr. med. Alessandro Ceschi, M.Sc., Chief of Medical Education and Research at General Directorate of the Ente Ospedaliero Cantonale (EOC) in Lugano, as the new President of the SCTO.
About Prof. Ceschi
Prof. Dr. med. Alessandro Ceschi, M.Sc. is a board certified clinical pharmacologist and toxicologist, and general internal medicine specialist physician. He currently serves as Chief of Medical Education and Research of the General Directorate of the EOC, which includes the Clinical Trial Unit (CTU-EOC). Additionally, he is Chief Physician of the Division of Clinical Pharmacology and Toxicology and Medical and Scientific Director of the Institute of Pharmacological Sciences of Southern Switzerland, both based at the EOC. He is professor at Università della Svizzera italiana (USI) and senior lecturer at University of Zurich and University of Basel.
Member of the SCTO Steering Board since April 2023, Prof. Ceschi will assume the role of President as of 1 September 2025. His wide-ranging responsibilities and experience reflect his strong commitment to advancing clinical research, medical education, and patient care across Switzerland and beyond.
The SCTO sincerely thanks Prof. Dr. med. Christiane Pauli-Magnus for her remarkable leadership and tireless dedication over the past seven years. Her vision has significantly shaped the SCTO and strengthened Switzerland’s position in the field of clinical research. The SCTO is pleased that Prof. Pauli-Magnus will continue to contribute her expertise as a valued member of the SCTO Steering Board.
We warmly congratulate Prof. Dr. med. Alessandro Ceschi, M.Sc., on his appointment and look forward to continued growth under his leadership.
New member of the Steering Board
In addition, the SCTO is pleased to announce that Prof. Dr. Jörg Goldhahn, Privatdozent at the Department of Health Sciences and Technology at ETH Zürich and representative of the Collège des Doyens (Deans of the Medical Faculties of the Swiss Universities), has been elected to the SCTO Steering Board, succeeding Prof. Dr. Primo Schär. All the other current Steering Board members have been re-elected.
The SCTO extends its sincere gratitude to all Steering Board members for their valuable contributions and continued support.
News
13 March 2025
Register for the SCTO Symposium 2025 | Bridging the divide: Integrating clinical trials with medical care
This year's SCTO Symposium focuses on bridging the gap between knowledge generated by randomised controlled trials and its application in clinical care.
Join us on Tuesday, 3 June 2025, 09.30–16.30 in Basel to explore how we can integrate clinical trials with everyday medical practice, focusing on evolving frameworks, adaptable study designs and robust data infrastructure.
Co-organised by the Department of Clinical Research, University of Basel and the Swiss Clinical Trial Organisation, this event promises to be an insightful discussion on how we can improve patient outcomes by bridging the knowledge-to-practice gap.
Learn more about the programme here and register today.
We look forward to welcoming you to this important event in Basel!
News
28 February 2025
PPI online course for SNSF IICT 2025 grant applicants
The Swiss National Science Foundation (SNSF) has opened its eleventh call for its Investigator Initiated Clinical Trials (IICT) programme. This programme supports clinical trials on under-researched topics that meet medical as well as social needs. One of the programme’s requirements is that grant applicants state in both their letter of intent (LOI) and their application how they actively involve patients and the public (PPI) in their research projects.
On 1 April 2025, the SCTO is offering an online course on these PPI requirements to researchers who would like to apply to the SNSF’s IICT 2025 programme. In the course, we will give participants examples and practical tips on how to successfully address the IICT’s PPI requirements. This online course is free of charge and will be held in English.
Register now for the online course.
Applicants must submit an LOI by 27 May 2025. Applications must be submitted by 4 November 2025.
- Programme and registration for the SCTO online course orgnised in collaboration with the SNSF (in English):
SNSF IICT 2025 Application: How to Successfully Address the PPI Requirement - More information on the IICT 2025 call
News
20 February 2025
ECRIN: International Clinical Trials Day (ICTD) 2025
The European Clinical Research Infrastructure Network (ECRIN) invites you to the International Clinical Trials Day (ICTD) 2025 | Rethinking Clinical Trials: Inclusivity in Practice on Tuesday, 20 May 2025 in Madrid, Spain.
The ICTD will answer the question: How do we progress from discussion to action in ensuring the appropriate populations are considered in the design of a clinical study? And more.
Learn more about the ICTD 2025 here.
News
4 February 2025
Regulatory Shake-Up: Key Changes Impacting Clinical Research
Insights into the new ICH GCP E6(R3) and beyond
The SCTO Forum 2025, held on January 29 in Bern, explored key updates to the ICH GCP E6(R3) guidelines, focused on improving flexibility, efficiency, and data integrity in clinical trials. 77 participants attended this engaging event. Presentations covered the rationale behind the revisions, real-world evidence, and quality by design.
Key Takeaways:
- Ongoing reflection is essential in decision-making.
- Researchers must engage with both GCP E6 and E8 guidelines.
- The revisions provide time for preparation and are set to improve trial practices.
Learn more about the SCTO Forum and download the presentations here.
News
24 January 2025
Positive Evaluation of the SCTO by the Swiss Science Council
The Swiss Science Council (SSC) has published its report, Research Facilities of National Importance (Art. 15 RIPA): Evaluation of the Funding Proposals for the ERI Period 2025–2028. This evaluation, commissioned by the State Secretariat for Education, Research, and Innovation (SERI), provides the foundation for SERI’s funding allocation.
The SCTO received a positive evaluation, reaffirming its key role as a research infrastructure in advancing Switzerland’s clinical research landscape. The report highlights the SCTO’s central role in optimising and harmonising processes, best practices, and methodologies in clinical research through its Platforms and support for Clinical Trial Units (CTUs).
Read this interesting report here.
News
20 January 2025
SCTO Forum 2025
Regulatory Shake-Up: Key Changes Impacting Clinical Research
Insights into the new ICH GCP E6(R3) and beyond
The SCTO Forum 2025 will take place on Wednesday, 29 January 2025 in Bern and will cover updates to the ICH-GCP E6(R3) guidelines, focusing on flexibility, efficiency, and data integrity in clinical trials. Topics include the rationale behind the revisions, stakeholder feedback, and implications for Swiss research, as well as quality by design and innovative trial designs.
This SCTO event is open to members of the SCTO Clinical Trial Unit (CTU) Network and to partner organisations.
News
3 October 2024
Online seminar: HRA ordinances – what has changed?
The SCTO is pleased to announce an upcoming online seminar, organised by the Regulatory Affairs Platform and Education Platform, focusing on the latest changes to the Human Research Act (HRA) regulations. The amended ordinances enter into force on 1 November 2024.
This one-hour seminar will provide an important update for all clinical research professionals and will offer key insights into the new regulatory changes.
The seminar will take place on Monday, 28 October, from 12.00–13.00.
The Zoom link for this seminar is available for download on the SCTO Platforms website.
Don't miss out on this valuable learning opportunity!
News
12 August 2024
Second round of HRO lunch sessions by the SCTO Education Platform
Between September and December 2024, four additional sessions will be offered, providing essential insights and practical knowledge for observational and further-use projects.
The sessions will focus the following topics:
- BASEC – A practical walk-through for HRO projects
- Mastering Consent: Key insights into general and informed consent for HRO projects
- Data sharing and open research data – details for HRO projects
- Sample management and biobanking – essentials for HRO projects
The sessions are held online as a Zoom meeting and offer plenty of time for you as researchers to ask questions.
Participation is free of charge and the sessions will be held in English.
Register here for this interesting sessions: Seminar series: Facts and pitfalls of observational studies – How to plan and conduct HRO projects
News
20 June 2024
Highlights of the SCTO Symposium 2024: Engaging presentations on data-driven clinical research!
The twelfth SCTO Symposium was dedicated to the topic "Working towards efficient clinical data-driven research in Switzerland" and took place in Lausanne on Tuesday, 11 June.
We had the chance to listen to insightful presentations on the present and future of data-driven clinical research, given by inspiring speakers from all over Switzerland. Each session led to a fruitful discussion between the speakers and the audience on these hot topics.
The Symposium was being organised by the SCTO and the Clinical Research Center (CRC) in Lausanne. 170 participants attended the event.
A short summary of the various presentations and the slides can be found at SCTO Symposium 2024.
News
19 June 2024
SwissPedNet's NextGen Research Day 2024 is now an official pre-programme of the annual pédiatrie suisse Congress!
On 5 June 2024, a motivated group of young Swiss paediatric researchers met in Sursee for the NextGen Research Day, an official pre-programme to the annual pédiatrie suisse congress. The NextGen Research Day is organised by the Swiss Research Network of Clinical Pediatric Hubs (SwissPedNet) with the support of the Swiss Clinical Trial Organisation (SCTO). 29 participants attended this event. The participants were resident pediatricians, senior pediatricians, master students, PhD students, postdocs as well as researchers from other related fields such as pediatric psychology or pediatric nursing.
The NextGen Research Day included several workshops where the young paediatric researchers were able to participate in small groups and learn from a professional in the field: A speed-dating session with experts and a scientific escape room where the participants had to solve a clinical research challenge. Over lunch and coffee breaks, there were many lively discussions between participants and with the experts.
This year was the first time, the NextGen Research Day was organised and communicated as an official pre-programme to the annual pédiatrie suisse congress on 6 June and 7 June 2024. We hope that the communication at the congress and the excellent quality of the 2024 event will help us to reach an even a wider audience for the NextGen Research Day 2025.
We thank Pfizer AG, Novartis Pharma Schweiz AG, AbbVie Inc. and Eli Lilly (Suisse) S.A. for their financial support.
News
18 June 2024
Horizons – The Swiss Research Magazine
The voices of those affected: The SCTO talks about its commitment to patient and public involvement (PPI) in clinical research.
Medical science could never progress without clinical trials. Too often, however, these trials ignore the needs of the patients themselves. All that is now about to change.
There is great value in patient and public involvement (PPI) in clinical research because patients living with a particular disease or condition have an understanding and knowledge that can help develop and improve healthcare.
However, Switzerland is still in the early stages of development of patient and public involvement. “If our trials become more patient-friendly, it might also become easier to recruit enough trial participants in future, and keep them on board too” (Sabine Rütti Roch, SCTO).
That's why the SCTO has developed different resources for patient representatives and clinical researchers, and aims to develop a platform that brings researchers and patients together.
Read this interesting article from Horizons Magazine, to which the SCTO contributed.
News
24 May 2024
SCTO Annual Report 2023
In 2023, the SCTO and its network continued their mission to enable high-quality clinical research. In our Annual Report 2023, you can read about all the important activities that took place in our different key areas.
Here are some of our highlights from 2023:
- Our CTU Network was involved in more than 2,000 clinical research projects and trained over 8,000 clinical research staff.
- SCTO Platforms developed and shared five new tools and six publications with the clinical research community.
- Patient and public involvement (PPI) was included in various Certificate of Advanced Studies (CAS) programmes, and the European Patients’ Academy on Therapeutic Innovation (EUPATI) Switzerland held its first patient expert training.
- Switzerland became a full member of the European Clinical Research Infrastructure Network (ECRIN), allowing the Swiss research community full access to ECRIN’s services.
- Over 400 participants attended our six events.
In the upcoming year, the SCTO will pursue its mission to the research community by fostering and intensifying collaboration between national research infrastructures, by strengthening the Clinical Trial Unit (CTU) Network, and by increasing the harmonisation of clinical research through tools, guidelines, and relevant services.
News
11 April 2024
Young Talents in Clinical Research
With the Young Talents in Clinical Research program, the Gottfried and Julia Bangerter-Rhyner Foundation and the SAMS encourage young medical doctors to start out in clinical research. For 2024, CHF 1 million is made available to finance protected research time and project grants for a consecutive research project. The submission deadline is 30 June 2024.
You can find the call on the website of the SAMS.
For additional funding opportunities for young clinical researchers, visit the Clinical Research Careers website.
News
27 March 2024
Survey by ECRIN about national registries for observational studies and sharing of individual participant data
Clinical trial registries are established tools widely used in clinical research. Registration rates of clinical trials have increased, however, there remains a substantial underreporting of observational studies. A survey across eight European countries belonging to the European Clinical Research Infrastructure Network (ECRIN) was conducted to assess the status of national developments with respect to registries for observational clinical trials. The SCTO actively contributed to this survey, which was additionally targeted at the needs and requirements for sharing of individual participant data (IPD) from clinical trials/clinical studies.
Six out of the eight countries included in the survey reported on national registries activities for observational or health studies. The authors of the survey suggested that a first step of improvement could be to integrate the national registries with ECRIN’s clinical research Meta Data Repository (crMDR), covering all primary registries of the World Health Organization’s International Clinical Trials Registry Platform (ICTRP) plus ClinicalTrials.gov, Pubmed and some repositories for sharing of IPD.
Read the complete study Survey by ECRIN about national registries for observational studies and sharing of individual participant data, Christian Ohmann et al., Zenodo, published on 25 March 2024.
News
19 March 2024
Online course for IICT grant applicants on how to successfully address the PPI requirement: Video and presentations available.
The Swiss National Science Foundation (SNSF) has opened its tenth call for its Investigator Initiated Clinical Trials (IICT) programme. This programme supports clinical trials on underresearched topics that meet medical as well as social needs. In both their letter of intent (LOI) and their application, grant applicants must address how they actively involve or plan to involve patients and the public (PPI) in their research projects.
For researchers who are interested in applying to the IICT 2024 programme, the SCTO together with experts of the SNSF, the Department of Clinical Research Basel (DKF Basel) and the Centre hospitalier universitaire vaudois (CHUV) organised an PPI online course on 18 March. In this course, we discussed why PPI is particularly useful when planning and developing clinical trials. We also provided practical tips and examples for an effective and meaningful approach to PPI including helpful tips on how to successfully address the PPI requirement. This online course was recorded, the video is available on our website.
News
15 March 2024
New paper published co-authored by SCTO: What are bottlenecks to health data sharing in Switzerland? An interview study.
Sharing health data for research requires the alignment of numerous factors, including regulation, access, interoperability, standardisation, and collaboration between researchers and patients. In Switzerland, like elsewhere, this process encounters various hurdles and bottlenecks, not only at the international level or with third parties but also within Swiss Universitiy Hospitals.
The recently published article, featured in Swiss Medical Weekly, sheds light on the legal, ethical, and technical challenges surrounding data sharing in Switzerland. The interview study was co-authored by our SCTO Regulatory Affairs Platform members Claudia Becherer and Elke Hiendlmeyer, in collaboration with several organisations including the Health and Policy Lab at ETH Zurich, the Swiss Personalized Health Network (SPHN), the Swiss Biobanking Platform and the Bern Center for Precision Medicine.
The majority of stakeholders surveyed in semi-structured interviews believe that the most complex and confusing aspects of data exchange do not lie in the actual data transfer itself, but in the associated processes and systems. The uncertainties relate to data protection laws, data ownership issues and processes for anonymisation and pseudonymisation. The study concludes that facilitating data access and exchange in Switzerland primarily requires further legal clarification, training and investment in sustainable infrastructures.
What are the bottlenecks to health data sharing in Switzerland? An interview study by Kelly E. Ormond et al., published on 22 January 2024 im Swiss Medical Weekly
Event
11 March 2024
SCTO Symposium: Working towards efficient clinical data-driven research in Switzerland
This year’s SCTO Symposium is dedicated to the topic «Working towards efficient clinical data-driven research in Switzerland» and will take place in Lausanne on Tuesday, 11 June.
The volume and complexity of data generated in clinical research have increased considerably due to several factors. The ways in which clinical trial data are generated, managed, and shared (data ‘’flows’’ in a clinical trial) have all become increasingly important for achieving efficient outcomes in both national and multinational trial settings.
Join us at the SCTO Symposium to navigate challenges faced by clinical researchers, the SCTO’s clinical trial units (CTUs), data centres, and biobanks in this current environment. We will address a range of related topics – including regulatory considerations, IT and infrastructure requirements, data governance, data sharing.
The symposium is being organised by the SCTO and the Clinical Research Centre (CRC) in Lausanne. It will be held in English.
Visit our event website for more information, we look forward to meeting you there!
Programme and registration: SCTO Symposium
Event
29 February 2024
Heads up young researchers in the field of paediatrics: An innovative event is just around the corner!
NextGen Research Day is an innovative training event for young researchers and doctoral students in the field of paediatric clinical research. The event is being organised by SwissPedNet, the Swiss Research Network of Clinical Pediatric Hubs. At the event, participants will find out what knowledge and skills are needed to conduct clinical studies and research projects, and they will get the tools they need to successfully run their projects.
When? 5 June 2024
Where? Cantonal Hospital Lucerne
Registration and further information NextGen Research Day 2024

Event
28 February 2024
SCTO online course for IICT 2024 grant applicants
The Swiss National Science Foundation (SNSF) has opened its tenth call for its Investigator Initiated Clinical Trials (IICT) programme. This programme supports clinical trials on under-researched topics that meet medical as well as social needs. One of the programme’s requirements is that grant applicants state in both their letter of intent (LOI) and their application how they actively involve patients and the public (PPI) in their research projects.
On 18 March, the SCTO is offering an online course on these PPI requirements to researchers who would like to apply to the SNSF’s IICT 2024 programme. In the course, we will give participants examples and practical tips on how to successfully address the IICT’s PPI requirements. This online course is free of charge and will be held in English. Please register online.
The portal mySNF is open for IICT submissions as of mid-April 2024. Applicants must submit an LOI by 27 May 2024. Applications must be submitted by 1 November 2024.
- Programme and registration for the SCTO’s online course (in English):
IICT 2024 Application: How to Successfully Address the PPI Requirement - More information on the IICT 2024 call

Event
22 February 2024
New SCTO Platforms seminar series: Facts and pitfalls of observational studies – How to plan and conduct HRO projects
The new lunch seminar series, developed by the SCTO’s Education Platform, provides a concise overview of the qualitative, regulatory, and legal requirements for conducting and planning observational studies (projects subject to the Human Research Ordinance (HRO)).
Four online sessions are scheduled to take place from March to May and are now open for registration. Please note that these sessions are free of charge and will take place in English.
More information and registration: Seminar series: Facts and pitfalls of observational studies – How to plan and conduct HRO projects
Event
22 February 2024
Second EUPATI CH patient training course
With patient and public involvement (PPI), patients and members of the public contribute their personal experience with a disease to a research project by actively shaping the project’s objectives and its design and by evaluating it.
In order to prepare individuals who are interested in participating in designing and evaluating studies as a PPI contributor, Switzerland’s national platform of the European Patients’ Academy on Therapeutic Innovation (EUPATI CH) and the Department of Clinical Research (DKF) at the University of Basel are offering, for the second time, their training course to become a EUPATI CH patient expert (held in German). The first course was successfully concluded in December 2023, and the second course will take place in April 2024 (registration deadline: 15 March).
More information is available (in German) at EUPATI CH.
Event
22 February 2024
D|A|CH symposium 2024: 16–17 September in Berlin
This year’s trinational symposium on clinical trials in Germany, Austria, and Switzerland (D|A|CH) will take place on 16 and 17 September in Berlin.
The fourth D|A|CH symposium features beginner and advanced programme sessions with over 50 speakers and experts from three countries. Topics include clinical trial regulation, patient involvement, patient education, decentralised trials, workforce shortages, data protection, medical devices, and much more. The SCTO is part of the programme committee, and members of the SCTO Network will participate as speakers at this year’s D|A|CH symposium.
This event will take place in German. Early-bird registration with a price reduction is open until 28 February.
For more information, the programme, and registration, visit the D|A|CH symposium’s website.

Event
2 February 2024
SCTO Forum 2024: Slides from presentations available online
During the SCTO’s annual forum on 31 January 2024, in Bern, we delved into the complex regulatory environment of clinical research and addressed the revision of the ordinances related to the Human Research Act (HRA). Together with our 78 participants, we explored the hot topics sparked by this revision that ignited insightful discussions.
PDFs of the slides from presentations are available on our website: SCTO Forum 2024.

Event
26 January 2024
SCTO Symposium 2024: Save the date
The SCTO Symposium 2024 will take place in Lausanne on 11 June 2024. Together with the Clinical Research Centre (CRC) Lausanne, the SCTO is holding this year’s symposium on the topic of working towards efficient clinical data driven research in Switzerland.
The event will take place in English. Make sure to mark your calendar for June 11 – registration opens soon!
News
26 January 2024
Article on Patient and Public Involvement in the Swiss medical bulletin Schweizerische Ärztezeitung
The below linked article published in the latest issue of the Swiss medical bulletin Schweizerische Ärztezeitung explores the participation of patients in the Swiss healthcare system.
The SCTO contributes significantly to Patient and Public Involvement (PPI) in academic clinical research. We support numerous PPI projects in Switzerland by providing trainings and developing PPI resources.
Read the article online: Teilhabe braucht einen Dialog auf Augenhöhe (Involvement Requires a Dialogue on Equal Terms) by Adrian Ritter, published on 24 January 2024, in the Swiss Medical Journal. (in French and German)
Learn more about SCTO PPI resources and PPI Mapping online.